The World Health Organization (WHO) made great strides in its recommendations on how cannabis should be scheduled, but the global industry believes there’s more work to be done.
Martin Jelsma, drugs and democracy program director, at the Netherlands-based Transnational Institute, emphasized to Marijuana Business Daily that there are “very positive elements in the WHO recommendations,” including:
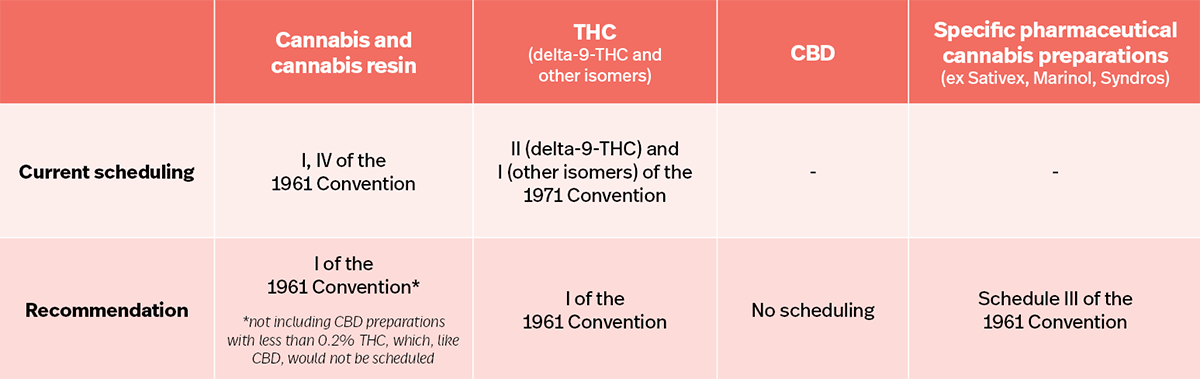
- Recognition of “medical usefulness” with its removal from Schedule 4.
- Additional clarity about CBD not being under international control.
- Resolution of the inconsistency of having cannabis under the 1961 and THC under the 1971 Convention.
But the recommendations – first reported by Marijuana Business Daily – didn’t go far enough, Jelsma said.
“(It’s) quite disappointing that the WHO recommends to keep cannabis in Schedule I,” he added.
Few people expected a really bold move, especially since it is the first time in nearly 50 years that cannabis classification has been considered by the international body.
“Complete descheduling of cannabis is more a political matter, not a scientific issue for WHO experts,” Pachta said.
The recommendations are nonbinding and must be voted on by the 53 member countries of the United Nations Commission on Narcotic Drugs (CND).
The earliest a vote could take place is in March, but a delay in the release of the recommendations may push the consideration to next year.
A government official from a member state who asked not to be identified told MJBizDaily that the CND chair is currently seeking input on whether to hold the vote this year.
 Validation for legalization
Validation for legalization
“It is auspicious that WHO has adopted a rational approach to cannabis and its compounds, incorporating advances that have long been accepted by the scientific community,” Diego Olivera, head of Uruguay’s National Drugs Council, told MJBizDaily.
“The recognition of medicinal uses and a more adequate assessment of the potential for abuse of THC are elements that support the processes of responsible legal regulation such as the Uruguayan case.”
Most view this as a positive step – albeit just a single step – forward for the blossoming industry.
“I am sure politicians and industry leaders will follow the WHO development closely as it will set the tone to a large degree for the future in the industry,” said Michael Prytz, investment manager for Invest in Denmark and senior adviser for Denmark’s Ministry of Foreign Affairs.
If the recommendations are followed, “more countries will establish medical cannabis programs, and proponents of medical use of cannabis and its derivatives will now have the possibility to refer to the obligation of parties to the 1961 Convention to make cannabis medicines available to patients,” Pachta said.
Key industry players also welcomed the recommendations – especially the clarifications around CBD.
“The WHO’s decision is another positive step forward for the global movement to end prohibition and the many harms it causes,” Brendan Kennedy, president and CEO of British Columbia-based Tilray, told MJBizDaily.
“By changing the international standard for the classification of CBD, the WHO is opening the door to governments to establish regimes that properly regulate CBD products.”
If the recommendations are accepted, business prospects around the globe could expand exponentially.
“It could turn the CBD-based products into a regular commodity and will open the world market,” said Inbar Maymon-Pomeranchik, founder of Israel-based Biodiligence and executive director of Ananda Developments.
“With far less limitations, products range will be unlimited. Once it will be possible, countries like Israel, with amazing technologies, will be able to export products in a very easy way.”
Greg Engel, CEO of Canada’s Organigram, agreed: “Does it become the next Omega 3? As the restrictions come off, it opens the door for that market to evolve quickly.”
Tempered reactions
Not everyone is as quick to embrace the recommendations as the bastion of hope.
Nathan Emery, a business executive from Lesotho, Zimbabwe and South Africa, noted that “the CND is a reactionary dinosaur in regards to cannabis and should probably be ignored – and definitely not tied to other UN assistance or censure, where developing countries would capitulate to their pressure points in developing a cannabis industry because they rely heavily on multilateral assistance.”
In addition, the Transnational Institute’s Jelsma worries about possible implications for medicinal preparations.
“On the one hand, the WHO notes that ‘preparations’ (defined as mixtures containing a scheduled substance) on the basis of treaty article 2.3, are subject to the same measures of control as the drugs which they contain, except for specific exemptions made for preparations under Schedule III,” he said.
But the WHO also recommends an exemption for THC as a component of a pharmaceutical preparation that “cannot be recovered by readily available means.”
“It appears that the WHO attempts to introduce a somewhat arbitrary and ill-defined distinction between products like Sativex and Marinol (which are specifically mentioned as examples) and other types of medicinal cannabis preparations,” Jelsma said.
Such an exemption could “favor specific products of the pharmaceutical industry over more natural oils/extracts without a clear rationale.”
A government official who asked not to be identified explained to MJBizDaily that a country could ship products in Schedule III to another country without asking for international quotas or import permits from the country of destination, simplifying international trade.
— With reporting by Matt Lamers in Toronto.





